Abstract
After over five years of planning and construction, two months old newly open Hospital Sultan Ism ail was infested with the deadly fungus. The areas of the extensive visible mold growth (Aspergillus fumigatus) were widespread in the entire hospital 10 story building which had 704 beds and 3004 rooms. A focused environmental investigation of microbial growth within the building followed, revealing moderate to high levels of fungi on interior surfaces and air sampling results showed >1000CFU/m3 in most areas. The mycological goal of the building restoration project was to reduce microbial reservoirs and the control of fungi on all exposed surfaces to the lowest feasible level. All visibly colonized materials in the building were discarded and all fine dust on interior surfaces was removed by vacuuming and/or damp wiping. A long-active organosilicon antimicrobial (BRShield™), 3-trimethoxysilylpropyldimethyloctadecyl ammonium chloride was selected to treat all surfaces throughout reconstruction. Re-evaluation of the facility at 5 months following restoration showed 12% of the indoor environment to be free of airborne fungi; 53% with <100 CFU/m3 of air and 35% with over 100 – 200 CFU/m3.
Introduction
There is growing concern about adverse health effects of fungal contamination on occupants of Hospital buildings since the nineteenth century. Then, as now, the morbidity and mortality resulting from microbial exposure encouraged the advancement of general disinfection, aseptic technique, and a variety of practices and procedures to control patient exposure to exogenous microflora. Fungal contamination in indoor environments has been shown to produce allergenic and toxigenic effects in occupants of this building.1, 2, 3 & 4
Control of microorganisms in indoor environments has traditionally focused on source control, ventilation, and air cleaning. Disinfecting compounds often are toxic. Harmful substances interfere with vital body processes by destroying enzymes, blocking oxidation, restricting the functions of various organs and initiate cellular changes and mutations. Ordinary cleaning can be effective in places that can be reached easily but the hard to reach places and the out-of-sight-out-of-mind places often become reservoirs and amplification sites for contaminating microbes. Cleaning also has limitations in its effectiveness and it is often expensive. This concern has encouraged researchers to look for other solutions.
Researchers in 1969 discovered a novel antimicrobial, using an alkoxysilane-coupling agent reacted to a quaternized amine, Plueddemann was able to covalently link directly to surface molecules. The bound monomers then reacted with each other to form a crosslinked polymer of extremely high molecular weight, thereby producing an essentially permanent antimicrobial surface 5, 6, 7, 8 & 9.
The modification of interior surfaces with a bound antimicrobial agent could prevent the development of microbial reservoirs in a building. The destruction of airborne microorganisms upon contact with antimicrobial surfaces would further reduce human exposure potential, producing an environment with a lowered risk of allergenic, infective, or toxigenic consequences for building occupants.
The present study was conducted to determine the level of microbial control possible from the comprehensive use of the material in a severely contaminated building and to assess the duration of effective control.
Materials and Methods
Background
This study was done over a 5-month period from November 2005 through March 2006 at the 10-story Hospital Sultan Ismail located in Johor, Malaysia. On September 25, 2004, the Ministry of Health ordered to close the doors of hospital after being hit by a deadly fungal infection. The contamination went throughout of this hospital. The remediation process and protective BRShield treatment began in March 2005 and was completed in four months.
Microbiological Sampling
Airborne fungal samples were obtained from 307 sites using Andersen air sampler loaded with Petri dish containing 20ml of Potato dextrose agar (PDA) at a constant sampling rate of 28.3 litres per minute. Air was impacted on the plates for one to four minutes at each site. Exposed plates were incubated at room temperature and examined over a five-day period. The number of colony-forming units per cubic meter (CFU/ m3) was calculated from the number of CFU’s counted and the collected air volumes. Samples were collected at designated intervals during pre and post-treatment.
Decontamination
The contaminated areas with open walls were decontaminated using a 20% Ox Bio+ and with the removal of residual spores after decontamination. Physical removal from vertical and horizontal surfaces was accomplished using disposable cleaning items. The surfaces had to be cleaned twice with frequent disposal of contaminated cleaning cloths. Ceilings were initially cleaned back and font using HEPA-filtered vacuum cleaning. All non-egress doors were taped shut after cleaning to help assure contamination control. Workers wore appropriate personal protection gear treated with the BRShield for added antimicrobial protection.
Surface Modification
All accessible interior surfaces (including ceilings, walls, above ceiling space, furnishings, elevator shafts, mechanical and electrical chases) were treated with the 3-trimethoxysilylpropyldimethyloctadecyl ammonium chloride (BRShield™ Antimicrobial) (6) in water in accordance with the manufacturer’s application specifications. The applications were randomly tested for uniformity and penetration throughout the treatment process using analytical methods appropriate for the active ingredient.
Results
The results of the samplings are presented to Table 1. Pre-treatment retrievals were in a range of 35 – 4730 CFU/m3 and the average sample concentration was 791.4 CFU/m3 during March 2005. The high levels of airborne fungal contamination were associated with the growth of visible mould. The intrusion of fungi from the outdoor environment was noted but the dominant and pervasive Aspergillus fumigatus was not part of this population.
| Location | Pre-treatment CFU/m3 |
2005 | 2006 | ||||
|---|---|---|---|---|---|---|---|
| Nov | Dec | Jan | Feb | Mar | |||
| Total Building | Average Sites |
791.4 307 |
48.1 307 |
56.4 307 |
72.2 307 |
101.4 307 |
96.6 307 |
| 1st Floor | Average Sites |
367.4 35 |
65.8 35 |
75.1 35 |
77.1 35 |
99.3 35 |
117.9 35 |
| 2 Floor | Average Sites |
533 38 |
55.1 38 |
71 38 |
54.5 38 |
111.2 38 |
111.8 38 |
| 3 Floor | Average Sites |
715.7 42 |
65.8 42 |
80.3 42 |
47.2 42 |
109.2 42 |
111.3 42 |
| 4 Floor | Average Sites |
502.8 42 |
79.4 42 |
59 42 |
89.6 42 |
91.4 42 |
102.4 42 |
| 5 Floor | Average Sites |
424.1 42 |
46.3 42 |
42.1 42 |
58.1 42 |
93 42 |
95.6 42 |
| 6 Foor | Average Sites |
1978.2 36 |
58.1 36 |
45 36 |
97.7 36 |
94.9 36 |
70.1 36 |
| 7 Floor | Average Sites |
738.5 24 |
32.3 24 |
29.3 24 |
66.5 24 |
90.3 24 |
121.6 24 |
| 8 Floor | Average Sites |
1336.8 24 |
30.8 24 |
41.1 24 |
65 24 |
96.3 24 |
87.3 24 |
| 9 Floor | Average Sites |
695 12 |
20.6 12 |
62.1 12 |
95 12 |
127.5 12 |
74 12 |
| 10 Floor | Average Sites |
622.1 12 |
26.5 12 |
59.1 12 |
71 12 |
100.7 12 |
74 12 |
Table 1. Concentration of air borne fungi (CFU/m3)
Post-treatment sampling during the first month following the restoration of the building produced an average of 48 CFU/m 3 at 307 sites. Retrievals were in a range of 0-178 CFU/m3. Of the sample sites, 307 sites 24% produced 0 CFU/m3; Figure 1 displays the level of reduction of airborne contamination in each floor during this evaluation period.

Figure 1 shows the reduction in microbial retrievals after treatment
Subsequently, routine air sampling performed in a one-month interval for another four months during December 2005 to March 2006 had an average of 56.4, 72.2 101.4 and 96.6 CFU/ m3 respectively (Fig. 2). The samplings produced retrievals in a range of 0-178 CFU/m3.
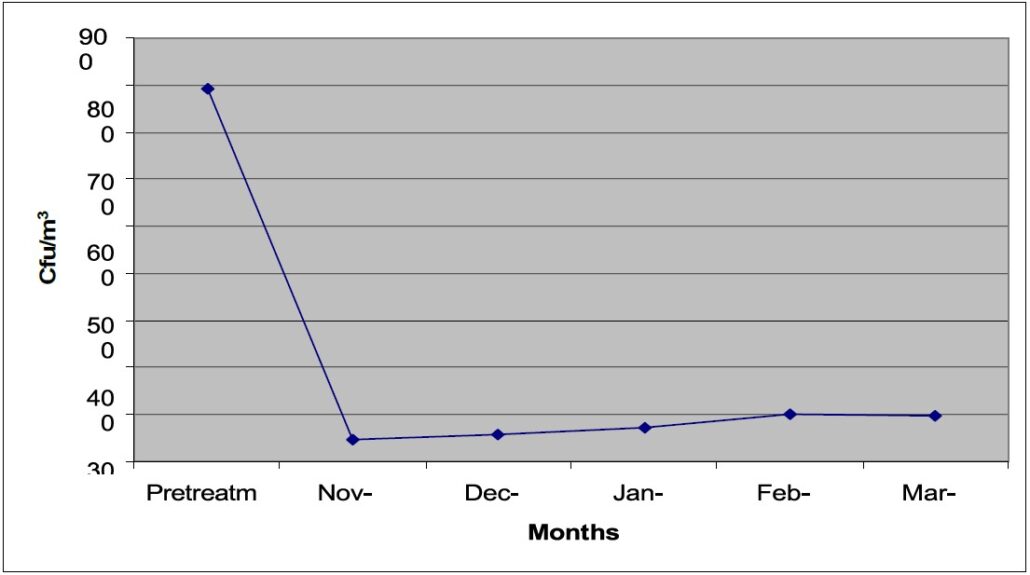
Figure 2 shows the retrieval averages of pre and post-treatment samplings in the building.
Swab sampling in March 2005 showed >300cfu/c m 2 fungi with Aspergillus fumigatus. Fogging with Ox Bio + followed by application of BRShieldTM demonstrated no Aspergillus fumigatus recovered from the horizontal and vertical surfaces. The absence of Aspergillus fumigatus in the post-cleaning phase assured that the contaminant was removed.
Discussion
The microbial colonization of interior surfaces in buildings is well known when extensive and often normal water damages occur during the construction period. Building construction is susceptible to water damage during storms if the exterior of the building is compromised or while new plumbing and fire management systems are being tested. Otherwise, water damage can occur due to high humidity or roof, plumbing and window leaks. These hidden inside the building constructions and colonize d by microorganisms have been suspected as a source of bioaerosol emission in so-called mould problem houses (10, 11 & 12).
Several studies have implicated airborne microbial contaminants in the development of Building-Related Illnesses and Sick Building Syndrome can be hazardous to compromised patients 13, 14, 15 & 16
Rhame et al (8) have shown a direct correlation between the concentration of airborne Aspergillus spores in hospital air and the incidence of aspergillosis among immunosuppressed patients; Arnow et al (9) report “ our findings strongly suggest that the inanimate hospital environment is a major determinant of the risk of endemic or epidemic nosocomial aspergillosis”;
All traditional disinfectant products have a vapor pressure, potentiating occupant exposure concerns; The duration of antimicrobial activity of traditional disinfectants is relatively short (ranging from a few minutes to several days) unless incorporated within a substrate with slow-release characteristics; Although most disinfectant formulations appear to possess increased activity against specific classes of microorganisms, this selectivity precluded broad-spectrum control. Many disinfectant active ingredients have been shown to give rise to an adapted microbial population.
Environmental sampling of both air and surfaces proved valuable for detecting and ensuring the removal of the potentially hazardous agent from the critical environment. The staffs were used to clean the rooms in a standard manner before occupancy. The final occupancy cleaning occurred some months after the environmental sampling certification of the contamination removal. On-going air-sample surveillance shows satisfactory air quality after occupancy.
Conclusions
With the continuing increase in the number of severely immunocompromised patients, hospitals are faced with the growing problem of invasive aspergillosis and other opportunistic fungal
infections. Since treatments of these infections are difficult and outcome is often fatal, preventive measures are of major importance in the control of invasive filamentous fungal infections. Data from the study indicate that surfaces modified with a silane antimicrobial (BRShield antimicrobial, SiQuat) provides substantive reduction of airborne microbial concentration even in extreme environmental conditions with sustained control of microbial levels and provides a cleaner, healthier environment of care. When viewed collectively, the safety, efficacy, and durability of this technology provide a unique opportunity to control the risks associated with microbial contamination in buildings without continuous care of the hard to reach surfaces.
Reference
- Miller, J.D., Fungi As Contaminants In Indoor Air, Proceedings of the 5 the International Conference on Indoor Air Quality and Climate, Indoor Air 90, Toronto, Canada, 7/29/90, pp. 51-64.
- Murry, W. A., Streifel, A.J., Odea, T.J., Rhame, F.S., Ventilation For Protection of Immune Compromised Patients, Ashrae, Transactions, 1988; 94(1):1185-1191.
- Kurup, V.P., Hypersensitivity Pneumonitis due to Sensitization with Thermophilic Actinomycetes, Immunology and Allergy Clinics of N.A., 1989; 9(2):285-306.
- Block, S.S. Microorganisms, Sick Buildings, and Building-Related Illnesses, Chemical Disinfection and Sterilization, 4th Ed., 1989, Chap. 65pp. 1107-1117.
- Speier, J.L. and Malek, J.R., Destruction of Microorganisms by Contact with Solid Surfaces, Journal of Colloid and Interface Science, 1982: 89: 68-76.
- Isquith, A.J., Abbot, A.E., and Walters, P.A., Surface-Bonded Antimicrobial Activity of an Organosilicon Quaternary Ammonium Chloride, App. Micro., 1972; 24: 859-863.
- Gettings, R.L., Personal communications with the author; and Gettings, R.L. and Triplett, B.L., A New Durable Antimicrobial Finish for Textiles, Book of Papers, AATCC National Conference, 1978.
- Hayes, S.F. and White, W.C., How Antimicrobial Treatment can Improve Nonwovens, American Dyestuff Reported, 1984.
- Kemper, R.A., Sustained Reduction of Aerobiological Densities in Buildings by Modification of Interior Surfaces with Silane Modified Quaternary Amines, Indoor Air Pollution, Chapter 5, 1991.
- Gravesen, S., J. C. Frisvad, and R. A. Samson. 1994. Microfungi, p. 49–50. Munksgaard Publishers, Copenhagen, Denmark.
- Johanning, E., R. Biagini, D. Hull, P. Morey, B. Jarvis, and P. Landsbergis. 1996. Health and immunology study following exposure to toxigenic fungi ( Stachybotryschartarum ) in a water-damaged office environment. Int. Arch. Occup. Environ. Health 68:207–218.
- Sorenson, W. G., D. G. Frazer, B. B. Jarvis, J. Simpson, and V. A. Robinson. 1987. Trichothecene mycotoxins in aerosolize d conidia of Stachybotrys atra. Appl. Environ. Microbiol. 53:1370–1375.
- White, W.C. and Kemper, R. A.,, Building Related Illness: New Insights Into Cases and Effective Control, ÆGIS Environmental Management, Inc., 1992; Form No. 4729-92.
- Kreiss, K., The Epidemiology of Building-Related Complaints and Illness, Occupational Medicine: State of the Art Reviews – Vol. 4, No. 4, Oct-Dec, 1989; pp. 575-592.
- WHO Reports, Biological Contaminants in Indoor Air, World Health Organization Meeting in Rutabaga, 1988; Euro. Report.
- Miller, J.D., Fungi as Contaminants in Indoor Air, Proceedings of the 5th International Conference on Indoor Air Quality and Climate, Indoor Air ’90, Toronto, Canada, Jul 29-Aug 3, 1990, pp. 51-64.
- Rhame, F.S., Streifel, A.J., Kersey, J.H. Jr., and McGlave P.B., Extrinsic Risk Fa actors for Pneumonia in the Patient at High Risk of Infection, Americ an J. of Med., 1984;
76: 42-52. - Arnow, P.M., Sadigh, M., Costas, C., Weil, D., and Chudy, R., Endemic and Epidemic Aspergillosis Associated with In-Hospital Replication of Aspergillus Organisms, J. Inf. Dis., 1991; 164: 998-1002.




